A Better Understanding of Adrenal Insufficiency for Medical and Reproductive Endocrinology, but Especially for Female Infertility
Very few among us still remember from our embryology classes in medical school that adrenal glands and ovaries share a common primordium (adreno-gonadal primordium, AGP), which arises from the intermediate mesoderm, a layer of the embryonic tissue that gives rise to the urogenital system. The AGP later splits into distinct primordia for the adrenals and gonads, which follow separate differentiation pathways. The mediodorsal portion of the AGP then separates and becomes the adrenal cortex and gonads, respectively, with the latter, of course, differentiating into either ovaries or testes.1
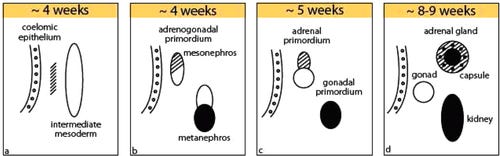
The developing ovary (i.e., the gonadal primordium) is then colonized by primordial germ cells that migrate in from the yolk sac.
Why is all of this of importance to reproductive endocrinology and infertility? In principle, for two reasons: First, adrenals and gonads (in this case, ovaries), of course, share in the production of steroid hormones with significant commonalities in steroid synthesis pathways.
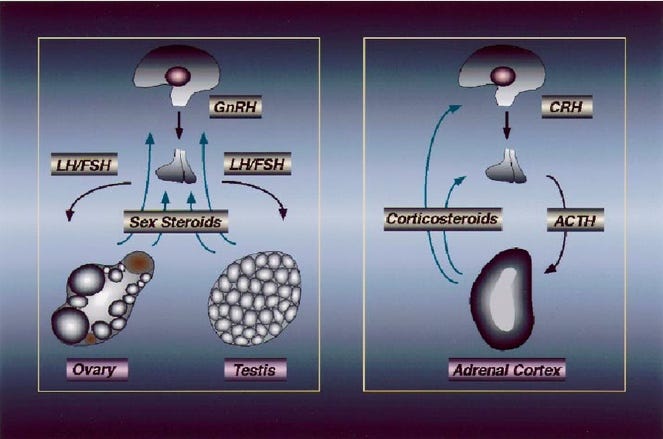
And as a relevant factor likely even more important for female infertility, adrenal glands and ovaries collaborate as a functional endocrine unit, a phenomenon the CHR’s researchers now have explored for over 20 years,2 in the process introducing androgen supplementation (with primarily dehydroepiandrosterone, DHEA) to the infertility field in cases of adrenal female hypo-androgenism which characterizes several important female infertility diagnoses.
What are these infertility diagnoses? They include premature ovarian aging (POA), physiological ovarian aging, primary ovarian insufficiency (POI), the rare end stage of POA before age 40,3 and—likely the most overlooked infertility diagnosis at all female ages—what under Rotterdam criteria has been described as Phenotype-D Polycystic Ovary Syndrome (PCOs) and investigators at the CHR, after age 35, have given the acronym HH-PCOS for Hyper/Hypo-Androgenic PCOS to denote that these women go from being hyper- to hypo-androgenic.4,5
This name stems from the observation that so-affected women post-menarcheal (in contrast to the classical description of this phenotype under Rotterdam criteria as normo-androgenic) are actually hyperandrogenic until roughly age 25, between ages approximately menarche and 25 demonstrate declining (adrenal) androgen production, by ca. age 25 enter the normal androgen range, and by approximately age 35 exit the normal range into clear hypo-androgenism. At this time, they usually become infertile and even treatment-resistant to in vitro fertilization.
This is one major distinction of this PCOS phenotype from phenotypes A, B, and C, which remain hyper-androgenic into advanced ages. A second distinction is that the D-phenotypes do not develop metabolic syndrome but, in contrast to the other three phenotypes, are in ca. 85% of cases associated with evidence of a hyperactive immune system (autoimmunity, inflammation, or just strong allergies), with ca. 45% of women demonstrating evidence of hypothyroidism and/or Hashimoto’s thyroiditis.6
And why is all of this important for adrenal insufficiency in general? There, indeed, are several very good reasons for such a conclusion:
(i) A first is that, as the CHR’s investigators have also pointed out to the medical endocrinology community, adrenal hypo-androgenism (as demonstrated by low DHEA-S in comparison to DHEA) raises a strong suspicion of adrenal insufficiency in general.7
Just recently, investigators indeed reported in the JCEM how accurate DHEA-S and baseline morning cortisol determinations together are in assessing adrenal insufficiency.8
(ii) Already noted, the current definition of adrenal insufficiency does not include insufficiency of the adrenal zona reticularis, which produces androgens,7 but adrenal insufficiency is generally considered an autoimmune condition, suggesting that this may also apply to insufficiency of the zona reticularis. If that is assumed to be the case, POA, POI, and HH-PCOS would have to be considered autoimmune conditions with adrenal epitopes as causes for these conditions, as already suggested by Bakalove et al in 2005,9 even though the ultimate effects are ovarian, which would explain why decades of searches for ovarian epitopes in association with POA and POI have remained basically fruitless. Only one such epitope has been described, and it appears only in patients with Addison’s disease, causing the very rare picture of autoimmune oophoritis.10
And in this regard, a very recently second paper published in JCEM reported that women with POI demonstrate a significantly increased prevalence of autoimmune diseases,11 with other autoimmune conditions more frequently being a typical characteristic of autoimmune diseases in general. And, on a side note, an increased association of POA has been reported by CHR investigators years ago.12
(iii) Finally, medicine in general only talking about the so-called hypothalamic-pituitary-adrenal axis. Isn’t it time to extend this axis—considering everything described here—to the ovaries, making it the hypothalamic-pituitary-adrenal-ovarian axis? We think so!
And here is what gave us the idea for this article: an otherwise very well-written recent review article in JAMA about adrenal insufficiency in adults, which, unfortunately, however, didn’t mention anything about what we just presented here.13
References
Xing et al., Endocrinol Metab Clin North Am 2015;44(2):243-274
Barad D, Gleicher N. Hum Reprod 2006;21(11): 2845-2849
Gleicher N, Barad DH. Reprod Biol Endocrinol. 2011;9:67
Gleicher et al., J Steroid Biochem Mole Biol 2017; 167:144-152
Gleicher et al., Endocrine 2018;59:661-676
Gleicher et al., Biomedicines 2022;10(7):1505
Gleicher et al., J Clin Endocrinol Metab 2017;102(9):3569-3570
Han et al., J Clin Endocriol Metab 2025;110(9):e3117-e3124
Bakalov et al., Fertil Steril 2005;84(4):958-965
Warren et al., Cell Mol Immunol 2014;11(6):510-521
Wang et al., J Clin Endocrinol Metab 2025;110(8):e2614-e2620
Sen et al., Nat Rev Endocrinol 2014
Vaidya et al., JAMA 2025;334(8):714-725


