Aging Through a New Lens: What Recent Research Reveals About Reproduction, IGF-I, Healthspan, and Cellular Renewal
We have repeatedly noted in these pages that the ovaries are the only organ in the human body that almost completely ceases to function prematurely. This makes ovarian aging uniquely relevant as a model for human aging more broadly. With the recent surge of interest in aging research, the field appears well-positioned for meaningful progress—both in understanding ovarian aging as a window into general aging, and in applying discoveries from general aging research back to the ovary.
With this in mind, we here present several articles for consideration.
Early menarche and childbirth accelerate aging-related outcomes and diseases
Anyone involved in biomedical or life-science research is by now familiar with the new publication model of eLife, a not-for-profit, peer-reviewed, open-access journal founded in 2012 by the Howard Hughes Medical Institute, the Max Planck Society, and the Wellcome Trust. Its creation followed a 2010 workshop at the Janelia Farm Research Campus.
In its current model, eLife no longer operates on the traditional accept/reject system. Instead, all papers selected for review are published as Reviewed Preprints. Although a formal acceptance rate is no longer applicable, only about 27–32% of submitted manuscripts are chosen to enter peer review. Following review, authors may request that their Reviewed Preprint be published as a Version of Record.
The study discussed here is one such Reviewed Preprint.
Researchers from the University of California, San Francisco, investigated what they call the “early-life reproductive phenotype”—simplified to age at menarche and age at first birth—and demonstrated that these early reproductive events influence health and longevity later in life. Their findings lend strong support to the antagonistic pleiotropy theory.
This theory holds that aging results from a declining force of natural selection with advancing age: biological traits that are advantageous early in life (such as early reproduction) may carry detrimental effects later in life. Although long debated, the theory has remained controversial.
Using Mendelian Randomization, the authors showed that later menarche and/or later age at first birth were genetically associated with:
Longer parental lifespan
Lower frailty index
Slower epigenetic aging
Later menopause
Reduced facial aging
Lower risk of several age-related diseases, including late-onset Alzheimer’s disease, type 2 diabetes, cardiovascular disease, essential hypertension, and chronic obstructive pulmonary disease
Using biobank data from nearly 200,000 individuals, the investigators found that menarche before age 11 and first birth before age 21 were particularly critical thresholds. Individuals meeting these criteria showed almost double the risk of diabetes and heart disease, and quadruple the risk of obesity.
The eLife editorial assessment confirmed the authors’ conclusion that the study provides “solid” supporting evidence for the antagonistic pleiotropy theory.
Reference
Xiang et al., eLife. https://doi.org/10.7554/eLife.102447.e
Why we should care about IGF-I levels and aging
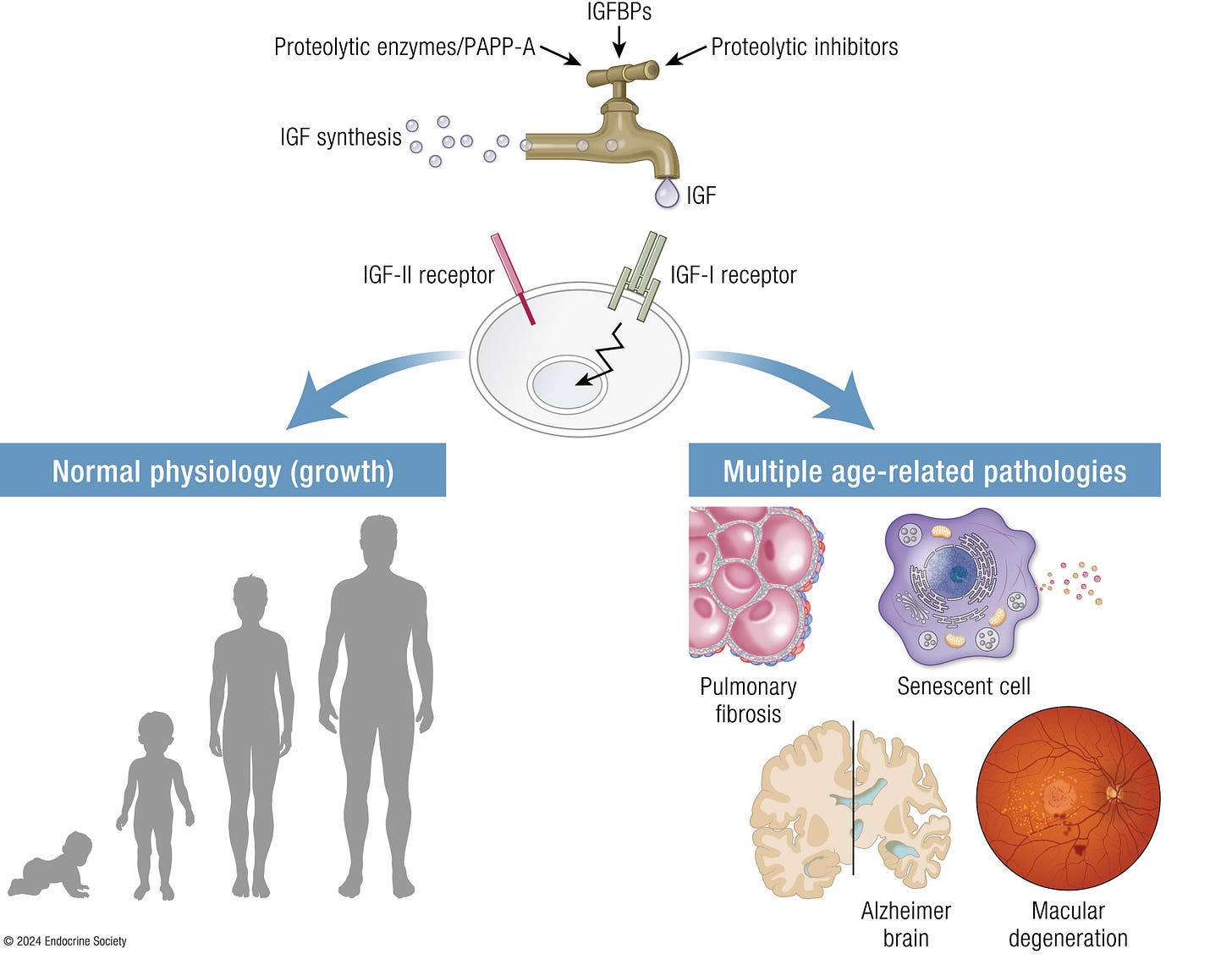
We are presenting here a recent paper from Endocrine Reviews on the IGF system and aging,¹ and one may wonder why. But the answer is rather straight forward: Human growth hormone (HGH) affects the early stages of follicle maturity through IGF-I and—though unknown by many—everybody who attempts to improve IVF outcomes through administration of HGH really stimulates IGF-I in the ovary in the hope that it will beneficially affect follicle growth and oocyte quality (and on a side-note, this is why HGH supplementation with normal IGF-I levels makes little sense!). What happens to the IGF system with aging, therefore, should, of course, be of some interest. And what we quickly found out in reading this paper was not only surprising but somewhat worrisome because IGF-I, in contrast to IGF-II, is not really a very “good” player in our bodies (see above the graphic abstract of the paper).
Pathologies associated with IGF-I
This excellent review article made three essential points:
(i) The IGF system is evolutionarily conserved and involved in fundamental aspects of aging.
(ii) It very well covered the involvement of the IGF system in cellular senescence, model organisms of aging, centenarian genetics, and 3 human age-related diseases—pulmonary fibrosis, Alzheimer’s disease, and macular degeneration.
And, finally, (iii) IGF system components, including receptors, ligands, binding proteins, proteinases, and inhibitors, may be disease drivers and potential targets in promoting healthy aging.
But what it means for reproductive medicine is that we, maybe, should be a little more careful in utilizing HGH in our patients (again, checking IGF-I levels before exposing a patient to HGH makes sense) because effects are somewhat mixed. Systemically, IGF-1 has been demonstrated in old mice to be important for spatial learning and memory. Low levels of IGF-I have been associated with neurodegenerative disorders, including Alzheimer’s disease. Concomitantly, high circulating levels in mouse models have been associated with beneficial effects on the aging brain and on Alzheimer’s.
References
Conover CA, Oxvig C. The IGF System and Aging. Endocrine Reviews. 2025;46(2):214-223. doi:10.1210/endrev/bnae029
Boosting the health and life spans
Among a series of recent papers on improving the health span as a method of improving longevity, a recent Medscape article by Daniela Ovadia attracted our attention¹ because it referred us to a still only online Review article in JAMA ahead of print by Stephen B. Kritchevsky, PhD, and Steven R. Cummings, MD, describing in what they called “a translational review” of the new discipline of “Geroscience.”²
To use their words, this new field of exploration aims to define and modify aging-related biological pathways, slow age-related disability, prevent age-related diseases, and increase disability-free survival. The hypothesis behind geroscience, moreover, suggests that biological aging must be viewed distinctively separate from chronological aging. However, disease-oriented prevention has been quite successful (consider vaccines, statins, etc.), but has, of course, considerable limitations, among those the fact that such an approach does not consider age-related health problems, like frailty and limitations in mobility, etc.
It has now become quite clear that a combination of certain cellular pathways influences lifespan (i.e., length of life) and a combination of other pathways may affect health span (length of life free from significant disease). And these pathways can be therapeutically approached. Both publications point out that the concept of caloric restriction has been the by far most extensively studied intervention in geroscience. Animal data demonstrating the benefit of moderate caloric restrictions appeared in the literature over 40-50 years ago.³ The exploding popularity of incretin therapies with semaglutide and tirzepatide has, however, now brought this issue to the attention of the general population.
Both of these medications have now been convincingly demonstrated not only to cause significant weight loss, but also to positively affect several major disease groups and, likely, life as well as health span. The same may also be the case for other already widely used medications, like metformin and rapamycin, with so-called senolytic drugs likely being the most aggressively investigated ones. Their function is to eliminate senescent cells in the body, which accumulate with advancing age and cause significant damage if not removed (see also below the discussion of Eric Topol, MD’s recent article on Ground Truths).
Geroscience, therefore, proposed a new paradigm for medicine: Instead of waiting for diseases to occur and then be treated to the best of our abilities, why not instead prospectively try to affect biological processes that increase an individual’s susceptibility? This is as far as we can go here in discussing the subject and the two publications, here the two initial references. We recommend reading both in their full length.
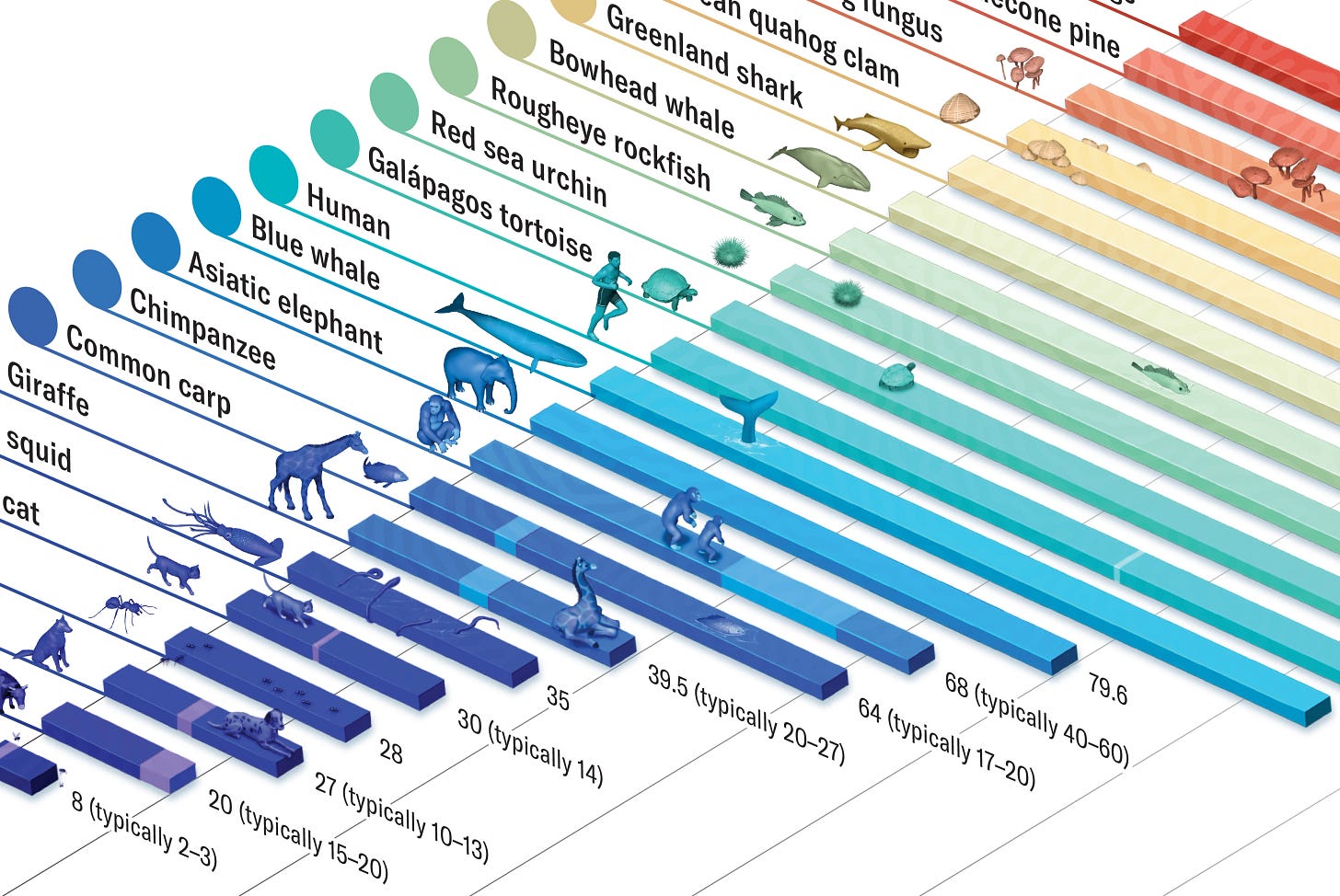
A relevant article also recently appeared in Scientific American. As an interesting exercise, it summarized the greatly varying lifespans of different species (see above), asking the question why they differed so much.⁴
And one, of course, cannot close on this subject without referencing Eric Topol, MD’s, recent Ground Truths article on the drivers of age-related disease,⁵ which, as most of his publications in Ground Truths, brilliantly summarizes the subject not only verbally but also graphically. He, for example, very well demonstrated how proteins from senescence cells in older people predicted age-related clinical outcomes in older patients (see the figure on the following page).
Going then in the article to a new epigenetic clock—one of his favorite subjects—“which connects the dots between aging, the immune system, inflammation, and lifestyle factors,” and apparently outperformed previously existing epigenetic clocks. We especially liked this section of his article because the CHR has by now, of course, for decades been emphasizing the importance of the immune system and of inflammation for female infertility. Topol then, moreover, also reported on recently published data demonstrating the importance of “immune resilience” for a person’s healthspan. Specifically, a signature of inflammation and/or immunosenescence aligned with a shorter lifespan, while less inflammation and immune system degradation resulted in longer life span. Unsurprisingly, he also noted that a recent study demonstrated that fast aging individuals were at increased risk for cognitive impairments, age-related diseases, disability, and mortality, and summarized the process and potential prevention in the two simple graphics to the left:
References
Ovadia D. Medscape. August 19, 2025.
Kitchevsky SB, Cummings SR. JAMA 2025; doi:10.1001/jama.2025.11289. Online ahead of print.
Weindruch et al., J Nutrition 1986;116(4):641-654
Moskowitz C. Scientific American, May 2025, pp. 94-95
Topol E. Ground Truths. June 8, 2025.
Can prostaglandin E2 (PGE2) rejuvenate stem cells?
This is what Stanford Medicine at Stanford University just reported:¹ Indeed, it happens to muscle stem cells in a mouse model after only brief exposure to PGE2, which is known to be involved in the body’s natural healing process after exercise or damage. The treatment apparently removes through aging-induced biochemical tags on the DNA of muscle stem cells that accumulate with aging and are passed on to daughter cells and hamper genes involved in muscle self-renewal, cell survival, and muscle cell function.
The article quotes Helen Blau, PhD, the senior author of a paper just published online in Cell Stem Cell² as saying that in the mouse model, “a single injection of PGE2 shortly after muscle injury caused an increase in muscle mass and strength two weeks later.” Moreover, the memory is apparently heritable, with the resulting improvement in stem cell function being inherited by the progeny of a cell, its cellular “children” and “grandchildren.”
These findings in a mouse model, of course, may have quite some clinical significance for human aging, muscle repair, age-induced—but also drug-induced—muscle loss (from weight loss drugs), and sarcopenia.
References
Conger G. Stanford Medicine. June 24, 2025. https://med.stanford.edu/news/all-news/2025/06/muscle-aging.html
Wang et al., Cell Stem Cell 2025;32(7):1154-1169