Is a New Area Of Extended Ovarian Stimulation Upon Us?
A guest contribution by Dr. Antonio La Marca and Dr. Maria Longo.
Antonio La Marca, MD, Professor of Obstetrics and Gynecology and Director of the OB/GYN Resident program, and Maria Longo, MD, are members of the Department of Medical and Surgical Sciences for Children and Adults, of the University of Modena and Reggio Emilia, Modena, Italy. La Marca can be reached directly at antonio.lamarca@unimore.it, and both authors can be reached through the editorial office of the CHRVOICE.
BRIEFING: In this very welcome Guest Contribution by our Italian colleague, Antonio La Marca, MD, we are confronted with a very logical concept, by the author appropriately given the name “extended ovarian stimulation,” which proposes that extended pre-exposure to LH hormone may improve the functional ovarian reserve, thereby improving the number of retrieved oocytes, which then automatically would also suggest an improved cumulative pregnancy and live birth chance for an IVF cycle.
This concept is of special interest for the CHR because a majority of CHR’s patients already receive at least 6–8 weeks of pretreatment prior to IVF cycle start with androgens (mostly DHEA) and, on rarer occasions, also pretreatment with human growth hormone. Addition of LH to this prep, therefore, would under current cycle protocols not further delay most patients in their IVF cycle starts. Seems like a great new idea to study.
Introduction
Controlled ovarian stimulation (COS) represents a cornerstone of assisted reproductive technologies (ART) and has traditionally been conceived as an intervention limited to the late, gonadotropin-dependent phase of folliculogenesis. Within this framework, ovarian response has been interpreted as the direct expression of a pre-existing, relatively fixed pool of recruitable antral follicles, commonly defined as the functional ovarian reserve. However, growing experimental, translational, and clinical evidence indicates that this pool is not static, but dynamically regulated by endocrine signals acting well before follicles reach the antral stage.¹
In recent years, particular attention has been directed toward the role of luteinizing hormone (LH) in early follicular development. Beyond its established function in steroidogenesis and final follicular maturation, LH appears to participate in the regulation of follicular progression at stages traditionally considered gonadotropin independent.²
These observations have paved the way for a novel therapeutic concept, referred to as extended ovarian stimulation, which aims to modulate folliculogenesis upstream of conventional COS to increase the number of follicles ultimately available for recruitment. This manuscript builds on the conceptual framework regarding the role of LH in early folliculogenesis and discusses the emerging clinical evidence supporting extended LH administration as a potential strategy to optimize ovarian response in patients undergoing infertility treatments.
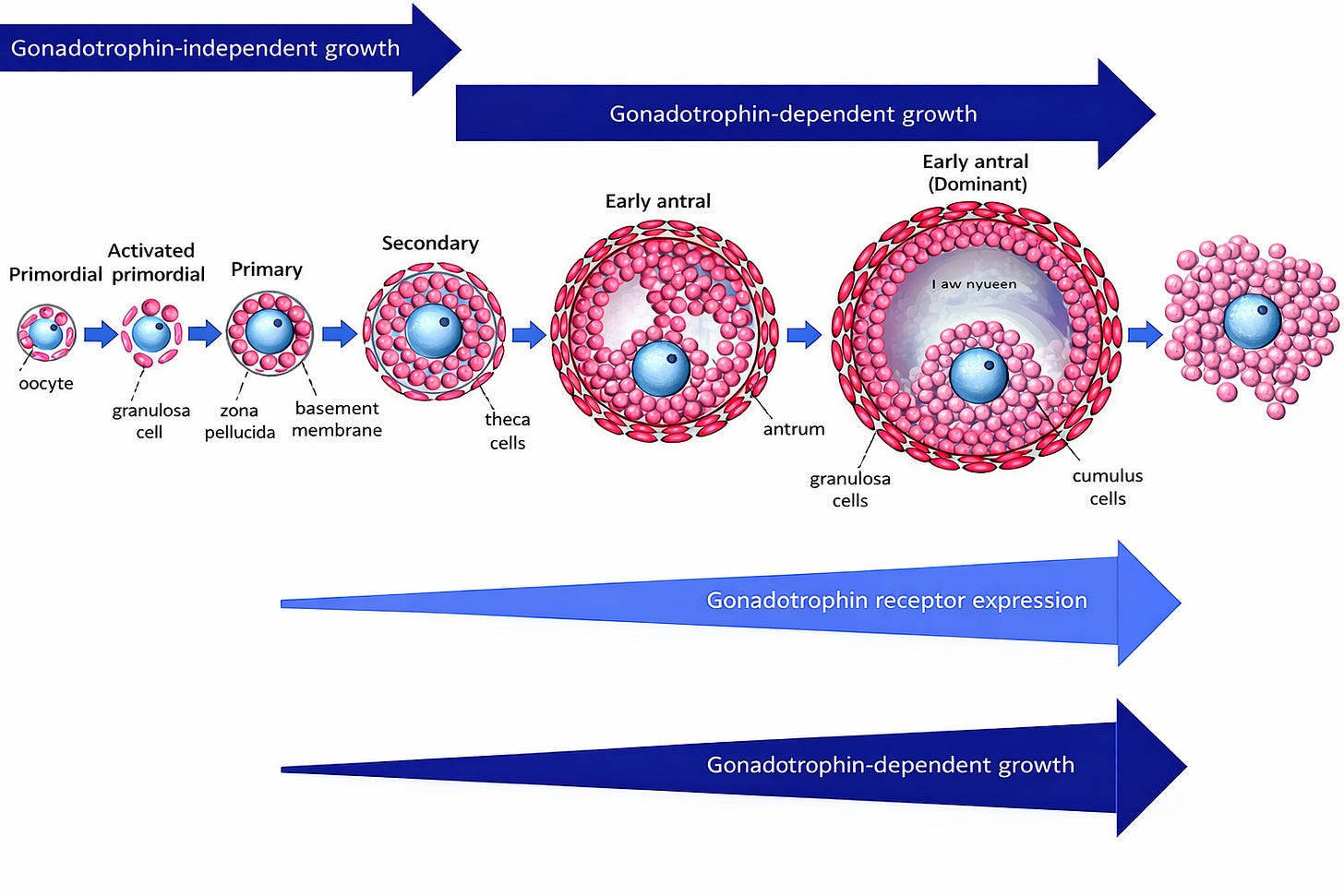
Revisiting Early Folliculogenesis: From Gonadotropin-Independence to Gonadotropin-Sensitivity
Human folliculogenesis is a prolonged and highly regulated process lasting approximately 300 days, from primordial follicle activation to ovulation. Classical models divide this process into two distinct phases: an early gonadotropin-independent phase, encompassing primordial, primary, and secondary follicles, and a later gonadotropin-dependent phase, beginning at the preantral–early antral transition. While this conceptual distinction has been useful, it is increasingly recognized as an oversimplification.
Multiple histological and molecular studies have demonstrated the presence of LH/human chorionic gonadotrophin receptors (LHCGR) in ovarian follicles much earlier than previously assumed. LHCGR expression has been detected not only in theca cells of antral follicles, but also in granulosa and theca cells of small preantral follicles, albeit at low levels.³ The functional relevance of these receptors is supported by in vitro follicle culture models showing that small preantral follicles require the combined presence of follicle-stimulating hormone (FSH) and low concentrations of LH to achieve optimal survival, growth, and antral formation.⁴
These findings support a continuum model of folliculogenesis, in which follicles progressively acquire increasing sensitivity to gonadotropins. In this context, LH may act as a permissive or accelerating factor, influencing the rate at which follicles progress from early growing stages to the antral pool, rather than functioning as an on–off switch restricted to late follicular development (see Figure 1).
Mechanistic Insights: LH, Androgens, and Follicular Progression
The biological effects of LH on early folliculogenesis appear to be largely mediated through its action on theca cells and subsequent androgen production. Androgens play a crucial role in early follicle development by acting on androgen receptors expressed in granulosa cells of preantral and small antral follicles. At physiological concentrations, androgens promote follicular growth, enhance follicle survival through anti-apoptotic pathways, and increase follicular sensitivity to FSH via up-regulation of FSH receptor expression.⁵˒⁶
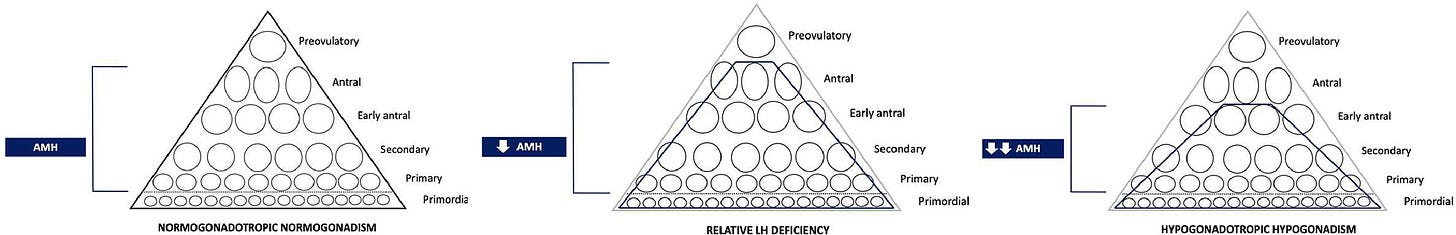
Experimental evidence suggests that LH-induced androgen synthesis facilitates the transition of follicles from the reserve pool into the cohort of growing follicles, thereby influencing the size of the functional ovarian reserve. In this setting, serum anti-Müllerian hormone (AMH), which is predominantly produced by preantral and small antral follicles, may be considered an indirect marker of the efficiency of early follicular progression rather than a pure reflection of the primordial follicle pool.⁷
Importantly, the effects of LH and androgens are highly context dependent. While physiological exposure appears to support follicular development, excessive androgen concentrations may disrupt folliculogenesis, leading to abnormal follicle accumulation and impaired selection, as observed in polycystic ovary syndrome. This underscores the need for carefully balanced LH exposure when considering therapeutic interventions targeting early follicular stages.
Clinical Models of LH Deprivation: Lessons From Abnormally Low Gonadotropins
Several physiological and iatrogenic conditions provide valuable in vivo models for understanding the role of LH in early folliculogenesis. Hypothalamic amenorrhoea (HA), pregnancy, long-term gonadotropin-releasing hormone (GnRH) analogue treatment, and hormonal contraception are all characterized by suppression of pituitary gonadotropin secretion and are consistently associated with reduced serum AMH concentrations and lower antral follicle counts (AFC).⁷˒⁸ Importantly, these changes are largely reversible following restoration of normal hypothalamic–pituitary activity, suggesting a functional impairment of follicular progression rather than irreversible depletion of the primordial follicle pool.⁹
HA represents a particularly informative clinical model. Although women with HA typically retain a normal primordial follicle reserve, a subset exhibits profoundly reduced AMH levels and AFC, especially in cases of long-lasting and severe gonadotropin suppression. This dissociation between chronological age, primordial follicle reserve, and functional ovarian reserve supports the hypothesis that chronic LH deficiency may slow follicular progression through early developmental stages, thereby reducing the pool of recruitable antral follicles.
Extended LH Administration: Clinical Proof of Concept
Based on this biological and clinical rationale, extended LH administration has been proposed as a strategy to increase the pool of recruitable antral follicles prior to conventional ovarian stimulation. The clinical feasibility of this approach has been illustrated by a small but highly informative case series involving women with HA and markedly reduced functional ovarian reserve.¹⁰
In these cases, recombinant LH was administered for a prolonged period (1–2 months) before COS at daily or alternate-day doses ranging from 150 to 187.5 IU. Following extended LH exposure, both patients demonstrated a clinically relevant increase in serum AMH concentrations and AFC, indicating an expansion of the pool of growing follicles. Importantly, this biological effect translated into improved ovarian responsiveness during subsequent in vitro fertilization (IVF) cycles, with a higher number of mature oocytes retrieved and the achievement of ongoing pregnancies. These observations provide compelling proof of concept that prolonged LH supplementation can modulate early follicular dynamics and enhance the functional ovarian reserve without directly acting on the primordial follicle pool (see Figure 2).
Implications for Assisted Reproduction
The concept of extended ovarian stimulation challenges the traditional temporal boundaries of COS. Rather than focusing exclusively on the final weeks of follicular growth, this approach aims to modify the trajectory of folliculogenesis months earlier, potentially redefining the management of patients with low ovarian response due to functional, rather than quantitative, deficits.
While current clinical evidence is limited to selected cases of profound LH deficiency, the implications may extend beyond HA. Subclinical LH deficiency, age-related changes in gonadotropin dynamics, or inter-individual variability in LH bioactivity could contribute to unexpectedly low AFC and poor ovarian response in otherwise eumenorrheic women. In these settings, extended LH administration may represent a novel adjunct strategy to improve ovarian responsiveness. However, several key questions remain unanswered, including the optimal dose, duration, and timing of LH exposure, patient selection criteria, and long-term safety.
What This Changes in Clinical Practice
Extended ovarian stimulation introduces the concept that reduced AMH levels and AFC do not invariably reflect irreversible ovarian depletion but may represent a functional and potentially reversible impairment of early follicular progression. In selected clinical contexts, such as hypothalamic amenorrhoea, prolonged gonadotropin suppression, or ovarian response unexpectedly discordant with age, targeted and prolonged LH supplementation before COS could represent a proactive strategy to enhance ovarian responsiveness. This approach encourages a shift from reactive management of poor ovarian response toward upstream modulation of folliculogenesis.
Conclusions
Accumulating experimental and clinical evidence supports a broader and more nuanced role for LH in human folliculogenesis, extending beyond late follicular maturation to include the regulation of early follicular progression and the determination of the functional ovarian reserve. Within this conceptual framework, extended ovarian stimulation, particularly through prolonged LH administration, emerges as a potentially transformative strategy aimed at reshaping the follicular landscape months before conventional COS.
Although current evidence remains limited and largely hypothesis-generating, it invites a rethinking of ovarian reserve interpretation and of the temporal definition of ovarian stimulation. If confirmed by prospective studies, extended ovarian stimulation may indeed inaugurate a new era in ART, characterized by earlier, more personalized, and biologically driven interventions designed to optimize ovarian response rather than merely accommodate its limitations.
References
La Marca A, Sunkara SK. Individualization of controlled ovarian stimulation in IVF using ovarian reserve markers: from theory to practice. Hum Reprod Update. 2014 Jan–Feb;20(1):124–140.
La Marca A, Longo M. New insights into the role of luteinizing hormone in early follicular growth. Reprod Biomed Online. 2023;47(1):102–110.
Takao Y, Honda T, Ueda M, et al. Expression of luteinizing hormone/human chorionic gonadotropin receptor in human ovarian follicles at different stages of development. J Clin Endocrinol Metab. 1997 Dec;82(12):3869–3875.
Wu J, Nayudu PL, Michelmann HW. FSH and LH requirements for growth and differentiation of preantral follicles in vitro. Endocrinology. 2000 Jan;141(1):195–203.
Sen A, Hammes SR. Androgens and follicular development: from physiology to pathology. Mol Cell Endocrinol.2010 Mar 25;315(1–2):1–12.
Gervásio CG, Bernuci MP, Silva-de-Sá MF, Rosa-e-Silva ACJS. The role of androgen receptor signaling in early folliculogenesis. Endocr Rev. 2014 Aug;35(4):671–694.
La Marca A, Grisendi V, Volpe A. Anti-Müllerian hormone concentrations in women with hypothalamic amenorrhea. J Clin Endocrinol Metab. 2006 May;91(5):1876–1880.
Sonntag B, Kiesel L, Nieschlag E, Behre HM. Anti-Müllerian hormone in women with hypogonadotropic hypogonadism. Hum Reprod. 2012 Jan;27(1):194–200.
Landersø SK, Forman JL, Birch Petersen K, Larsen EC, Nielsen HS, Nyboe Andersen A. Ovarian reserve markers after long-term suppression: evidence of reversibility. Hum Reprod. 2020 Jan;35(1):195–204.
La Marca A, Longo M, Grisendi V, Argento C. Extended luteinizing hormone administration increases functional ovarian reserve in women with hypothalamic amenorrhoea. Hum Reprod. 2022;37(6):1234–1242.