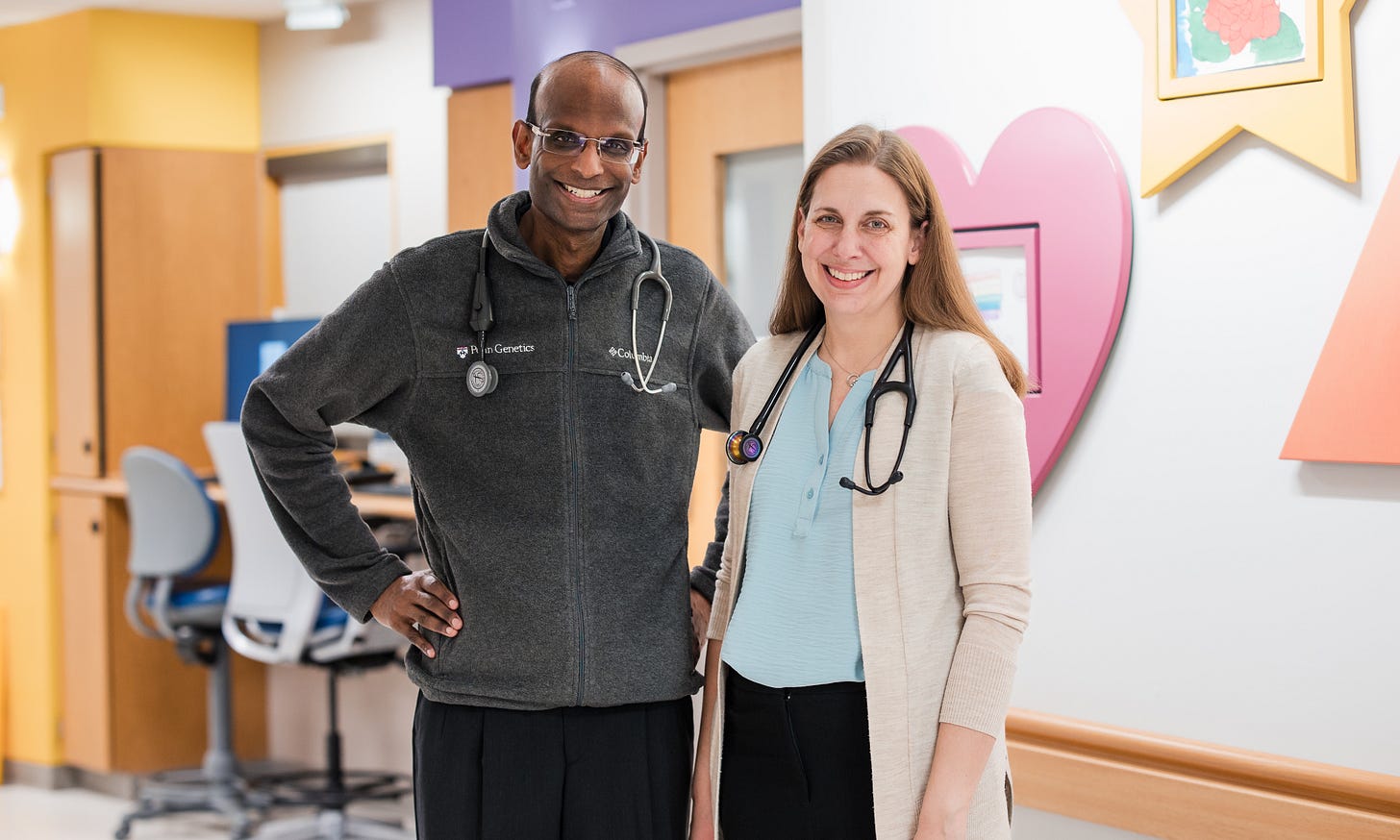Nature Magazine’s 10 People Who Shaped Science in 2025, and the 3 We Chose Among Them to Feature Here
By Norbert Gleicher, MD, Medical Director and Chief Scientist, at The Center for Human Reproduction in New York City. He can be contacted directly at ngleicher@thechr.com.
The CHR’s Medical Director and Chief Scientist, Norbert Gleicher, MD, here discusses three out of the ten science events Nature Magazine selected for having “shaped” science in 2025. Though radically different events, all three—as will quickly become apparent—involved special individuals in very special circumstances. And, though not directly related to reproductive medicine and/or infertility, they all have significant relevance and, even more importantly, they all teach lessons on how to improve and advance medicine.

As every year, Nature published in December its annual list of who the magazine’s editors considered to be the ten most important people who in that year had “helped shape science.” Such a choice, of course, by definition will always be—at least in some ways—biased, because choosing the top ten contributions to science in all of its fields is, of course, an insurmountable task.
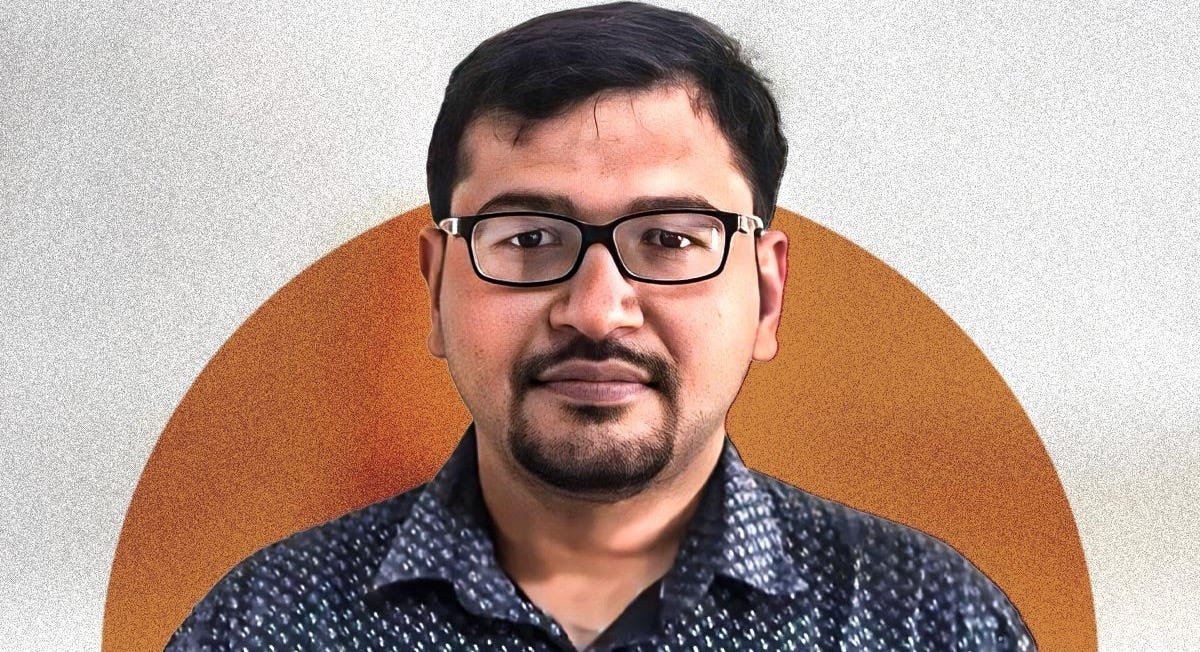
We, therefore, here do not present the complete list of all ten finalists that Nature magazine named, but decided to add our biases to theirs by selecting only three out of their ten whose deeds we really find exceptional. The first name to make it onto this mini-list did so not because of the science he produced, but because of how he protected science in general. It is Achal Agrawal, PhD, who in 2016 earned his PhD in applied mathematics from the University of Paris-Saclay, at which time he returned to India with the intent to work in education. But this is where his problems started.
As widely reported, what changed his life was a single encounter with an undergraduate student who had approached him with great enthusiasm—as Miryam Naddaf reported in his Nature profile¹—to discuss a planned research project and, in the conversation, revealed that he previously had used software “to paraphrase previously published work.” He “was shocked” when the student disputed that this represented plagiarism, and when he found out that this work had passed plagiarism checks by the university.
Recognizing how ingrained research misconduct had become in Indian science, he, in 2022, left his academic position a month after the encounter with the student and has since, without holding a salaried academic position, functioned as a freelance data scientist in Raipur,² dedicating himself to cleaning house. In doing this, he launched what is called the India Research Watch (IRW), an online group of researchers who highlight research misconduct of various kinds.
As Naddaf’s article pointed out, this year his (and, of course, other people’s) efforts led to a significant change in how the Indian government ranks higher education institutions, moving away from a primary emphasis on publication numbers. But as her article also noted, his activities have come at a high price to him, as he has been unable to find employment and now also has to fight a lawsuit brought against IRW by a private university.
Aware that cleaning up India’s science will take time, he is, nevertheless, committed to continuing to expose how flawed incentives in academia fuel research misconduct of all kinds. And India, as we have pointed out in these pages repeatedly before, is, of course, not alone in urgently needing such a “clean-up.” All of medicine and all of science publishing, including, of course, the infertility field, require a thorough clean-up of the publishing process. And this is likely the primary reason why Nature chose him among the ten finalists for 2025.
Our second selection among those ten is Israeli scientist Yifat Merbl, PhD (Weizmann Institute of Science in Rehovot)—the “peptide detective,” as the author of her biography in Nature, Cassandra Willyard, described her.³ And we chose her not only for her truly amazing scientific discoveries (which one day may indeed win her the Nobel Prize), but for two additional reasons which make her history really special—but let’s start at the beginning, and that is her research.
As Willyard described it, Merbl “simply found a new facet of the immune system hiding in cellular rubbish,” which will have—so far still almost unimaginable—diagnostic as well as therapeutic consequences for human existence and, of course, medicine. Investigating cellular recycling centers known as proteasomes, Merbl and her team discovered a completely new part of the immune system. Proteins enter these proteasomes and are shredded there before exiting as smaller peptide fragments. Puzzled by the complexities of these peptides, the team discovered, using public databases, that many of these fragments matched peptides with already known functions.
And, lo and behold, there were many functional matches, among them many known to obliterate bacteria. They identified approximately 1,000 fragments which likely are antimicrobial (imagine the potential therapeutic consequences at a time when the world—because of increasing bacterial resistance—is running out of well-functioning antibiotics). Her scientific achievement is therefore quite amazing and very much warrants her presence among the 2025 top ten.
But, as promised, there are two very interesting additions to her story. First, she makes no secret of the fact that, as a child, she was severely affected by attention-deficit/hyperactivity disorder and did not graduate from high school with her peers. As Willyard reported, Merbl, over the years, learned to accept “how her brain worked” and understood that it indeed gave her an advantage by often giving her “a different perspective” (what we here at the CHR over the years have come to call “to think differently”). Her doctoral adviser, later at Harvard Medical School, noted, according to Willyard, that “she liked to embark on scientific fishing expeditions, not knowing what she may catch, and made some terrific discoveries.”
And here is the second item that makes her story so remarkable. We noted in one of last year’s issues of the CHRVOICE that some buildings at the Weizmann Institute in Rehovot (Israel’s Harvard) were destroyed by Iranian rockets during the 12-day direct war between Israel and Iran. An Iranian missile strike indeed destroyed Merbl’s lab. We now quote verbatim how Willyard described what happened next:
“Merbl, who lives on campus, waited out the attack in a bomb shelter, then rushed to her lab. The building next door was on fire, and the power was off. She made her way through the building, navigating broken glass while wearing flip-flops, and closing freezer doors to keep samples cold.”
She and her team now have a new lab and are ready to keep looking for other secrets hiding in proteasome-produced peptides, convinced that the proteasome peptide story does not end with antimicrobial uses and that this is “not the end of the story yet.”
Which brings us to the third selection, which again involves something completely different, and not always very popular, namely gene-editing therapy. As reported by Heidi Ledford in Nature,⁴ it involved what she called the first-ever hyper-personalized CRISPR gene-editing therapy in the world, given to a six-month-old newborn who, after birth, was diagnosed with an extremely rare single-gene disease called carbamoyl-phosphate synthetase 1 (CPS1) deficiency, which impairs the ability to process proteins.
Under normal circumstances, the body routinely breaks down proteins, resulting in the production of ammonia. Ammonia is toxic and therefore—after processing in the liver by certain enzymes—is excreted in urine. An individual with CPS1 deficiency lacks one of these required liver enzymes. Consequently, ammonia accumulates in the blood, which can damage the brain and, in approximately half of cases, leads to death in infancy. The only treatment so far has been a liver transplant.

A pediatrician at Children’s Hospital of Philadelphia, Rebecca Ahrens-Nicklas, MD, PhD, and a cardiologist, Kiran Musunuru, MD, PhD, MPH, ML, MRA, at the Perelman School of Medicine at the University of Pennsylvania in Philadelphia, had a different idea. As earlier reported in The New England Journal of Medicine (and at the time discussed in the CHRVOICE), this diagnosis led to the first-ever so-called hyper-personalized CRISPR genome editing using base editing,⁵⁶ which attempts to correct one abnormal mutation (as Ledford noted, “one faulty DNA letter out of 3 billion in a human genome”).
To produce the gene-editing components to treat the infant was expected to take 18 months; it was achieved in six months (Integrated DNA Technologies, Coralville, Iowa). Born in August 2024, the infant received the first of three infusions in February 2025. After the first 307 days of life in the hospital, the male infant was discharged home. His tolerance for protein in the diet has improved, but he still needs medication and regular monitoring of ammonia levels. He is, however, home, hitting all developmental milestones, and was expected to make his first steps soon when Ledford’s article appeared.
This case was a watershed moment for genomic editing, comparable to the announcement of the first successful IVF cycle and the birth of Louise Brown in the UK on July 25, 1978, at 11:47 p.m. Both events represent moments in medicine where previously unimaginable medical interventions succeeded for the first time. Just as the birth of Louise Brown did not immediately result in hundreds of IVF babies, this first hyper-personalized case of CRISPR gene editing will not immediately result in an onslaught of additional successful genome edits. But look where IVF is today—and progress will be even quicker with gene editing, which is at least conceptually and technically a much simpler step than IVF.
It currently costs millions of dollars to treat one person with the kind of gene editing this infant experienced. But nobody ever calculated the costs of the Brown baby. If Patrick Steptoe, Robert Edwards, and the often-forgotten Jean Purdy had added up the costs over many years and large numbers of earlier failures, it likely would also have reached into the millions. And while IVF is not cheap today (and often still not covered by medical insurance), the costs are manageable for society, and the same will happen with gene editing.
One more issue must be mentioned, and it is an important reason why this medical achievement is among the three we chose: the relevance of what happened to this newborn has major potential significance for single-gene defects in human embryos during IVF. If a six-month-old newborn can be successfully treated in his germline, imagine how much simpler it must be to treat, for example, a six- to eight-cell cleavage-stage embryo or even a day-5 blastocyst.
References
Naddaf M. Nature. 2025;648:518.
Chakrabarty R. India Today Education Desk. December 23, 2025.
https://www.indiatoday.in/education-today/news/story/achal-agrawal-is-on-natures-2025-list-for-taking-on-research-plagiarism-in-india-2840487-2025-12-23Willyard C. Nature. 2025;648:527.
Ledford H. Nature. 2025;648:528–529.
Musunuru et al. N Engl J Med. 2025;392:2235–2243.
News release. The Children’s Hospital of Philadelphia. May 15, 2025.
https://www.chop.edu/news/worlds-first-patient-treated-personalized-crispr-gene-editing-therapy-childrens-hospital